Summary
Detail
Required documents
|
No |
Type of documents |
Note |
|
1 |
For goods transported by road, rail, and air: cargo medical declaration, road, rail, air transport, cargo medical inspection/handling certificate goods and means of transport by road, railway, or air (if any); |
01 |
|
2 |
For goods transported by water: a copy of the cargo manifest; certificate of medical examination of goods (on board ships), boats (if any). |
01 |
|
3 |
Application form: In case the medical declarant requests medical examination and treatment of goods in order to issue a certificate of medical inspection/handling of goods. |
01 |
Process Steps
|
Step 1 |
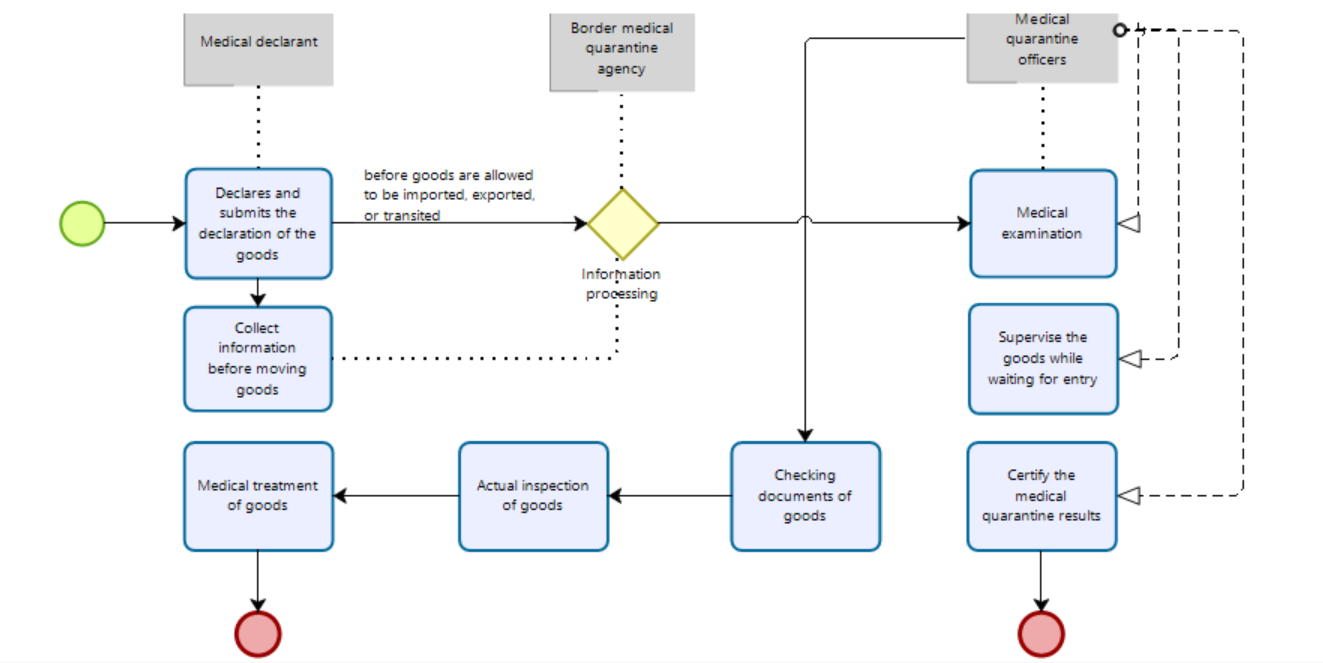
Medical declaration for goods: 1. For goods transported by road, rail, or air: the declarant declares and submits the medical declaration of the goods, the certificate of medical inspection/handling of the goods (if any) to the border medical quarantine agency or through the National Single Window Portal before goods are allowed to be imported, exported, or transited. 2. For goods transported by sea: the declarant shall declare and submit a copy of the goods declaration and the certificate of medical examination of the goods (if any) to the border health quarantine organization or through the National Single Window Portal 12 hours before the goods are expected to be imported, exported or transited. |
|
Step 2 |
Collect information before moving goods across borders
|
|
Step 3 |
Information processing for goods
|
|
Step 4 |
Checking documents of goods The medical examiner checks the following documents:
|
|
Step 5 |
Actual inspection of goods The medical quarantine officer requires the goods to be brought into the medical inspection area, and inspects the following contents:
- Means of transport passing through a country or territory with recorded cases of infectious diseases that the Ministry of Health requires to monitor; - Means of transport carrying sick patients or people suspected of having diseases or carrying pathogens of infectious diseases; - Means of transport carrying goods carrying pathogens of infectious diseases or suspected of carrying pathogens of infectious diseases.
|
|
Step 6 |
Medical treatment of goods Based on the results of the actual inspection, the medical quarantine officer may apply one or more of the following measures:
|
Process map:
Online service can be processed at:
https://dichvucong.gov.vn/p/home/dvc-chi-tiet-thu-tuc-hanh-chinh.html?ma_thu_tuc=2.000981
Online service can be processed at:
https://dichvucong.gov.vn/p/home/dvc-chi-tiet-thu-tuc-hanh-chinh.html?ma_thu_tuc=2.000981








